Valacyclovir indications and dosage in dermatology
Indications
- Episodic herpes labialis.
- Herpes labialis suppression.
- Herpes simplex prophylaxis after laser resurfacing.
- Varicella.
- Herpes zoster.
- Herpes zoster ophthalmicus.
- Primary and recurrent herpes simplex.
- Recurrent erythema multiforme.
- Suppression and episodic treatment for herpes simplex or varicella-zoster infections in HIV.
- Other subsets of herpes simplex infections.
Dosage
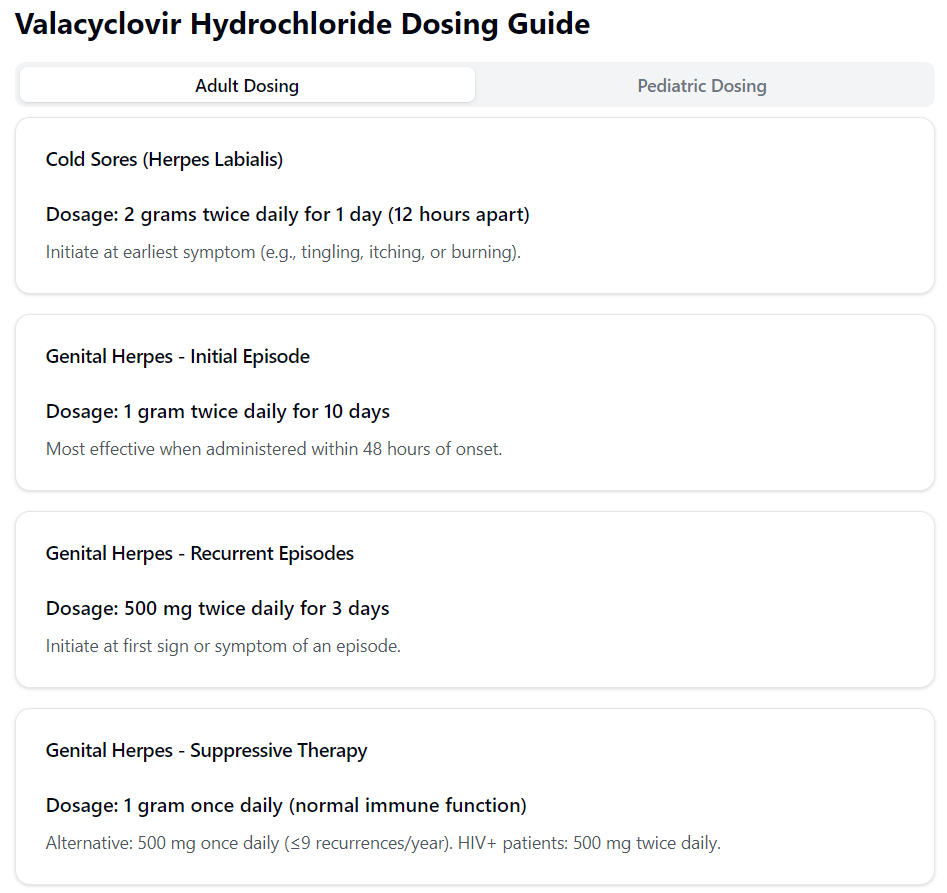
- Episodic herpes labialis: 2 g po bid for 1 day.
- Herpes labialis suppression: 500 mg po qd.
- Prophylaxis of Herpes simplex after laser resurfacing: 500 mg PO bid for 14 days ( starting one day before the procedure or on the same day)
- Herpes zoster: 1000 mg po q8h for 7 days.
- Herpes zoster ophthalmicus: 1 g po tid for 7 days.
- Primary genital herpes: 1000 mg po q12h for 10 days.
- Recurrent genital herpes: 500 mg po q12h for 5 days.
- Suppression and episodic treatment for herpes simplex in hiv: 500 mg po bid, 1000 mg po bid for 5 days.
- Suppressive genital herpes: 1000 mg once daily.
- Varicella: 1 g po tid for 7 days.
Renal dosing :
•Creatinine clearance (crcl) 50–90 ml/min: 1 g po q8h.
•Crcl 10–50 ml/min: 1 g po q12h–q24h.
•Crcl < 10 ml/min: 500 mg po q24h.
•Hemodialysis: 500 mg po q24h after dialysis.
•Continuous ambulatory peritoneal dialysis: 500 mg po q24h.
•Continuous arteriovenous hemofiltration: 1g po q12h–q24h
The efficacy of Valacyclovir in children below the age of 12 years has not been evaluated.
Mechanism of action
- Valacyclovir is converted into acyclovir, with increased bioavailability.
- It requires phosphorylation by viral thymidine kinase to be activated.
- The active triphosphate metabolite of acyclovir inhibits viral DNA polymerase and thus DNA synthesis.
Side effects
Common :
- Nausea, vomiting, diarrhea and headache.
- Agitation, vertigo, confusion, dizziness, edema, arthralgia, sore throat, constipation, abdominal pain, rash, weakness and/or renal impairment (infrequent).
Rare :
- Coma, seizures, neutropenia, leukopenia, tremor, ataxia, encephalopathy, psychotic symptoms, crystalluria, anorexia, fatigue, hepatitis, Stevens–Johnson syndrome, toxic epidermal necrolysis and/or anaphylaxis.
Contraindications
- Hypersensitivity to drug/class or acyclovir
Interactions
- Decreased rate of conversion of drug to acyclovir: cimetidine, probenecid.
Pregnancy &Lactation
- Pregnancy category: B.
- Lactation: Drug excreted in breast milk; use with caution.
Precautions
- Use with caution in patients with renal impairment, the elderly, and/or patients receiving nephrotoxic drugs.
- Central nervous system (CNS) effects may occur ; risk is higher in elderly patients.
- Thrombotic thrombocytopenic purpura (TTP)/hemolytic uremic syndrome (HUS) reported in patients with advanced HIV disease.
- Adequately hydrate patient; decreased precipitation in renal tubules may occur.
- Low dose (1000 mg/day) valacyclovir therapy may cause thrombotic thrombocytopenic purpura in an immunocompetent patient.
Drug Info
- Valacyclovir is an oral prodrug of acyclovir.
- In the oral form it is nearly as potent as intravenous Acyclovir.
#Valaciclovir #Valacyclovir #Valacyclovir dosage #Valacyclovir in dermatology #Valacyclovir indications #Valacyclovir side effects